If you like your chemistry colourful, dyes and indicators are worth learning more about, but can an old spice learn new tricks, or is it just a food fad? Yet again, we come up against questioning the bioavailability of compounds and learn about scientific studies that debunk some myths.
(This post has been prepared by students from the Theory and Modelling in the Chemical Sciences Centre for Doctoral Training, who are currently doing their PhD research in Oxford, Southampton and Bristol.)
1. The main image shows yellow turmeric powder, along with one of its key components, a molecule of curcumin which gives rise to the yellow colour, and a number of different items of common laboratory glassware (left to right: conical flask, beaker and round-bottomed flask). Turmeric can either be used fresh, or as a powder, which is produced by grinding the cooked and dried rhizome of a member of the ginger family, Cucurma longa, grown in the warm, moist climates of southern Asia. It is a common household spice and a major ingredient in many curry powders. The plant itself originates from India and is now widely grown in many Asian countries for use not only in food but also in dyeing and (traditional) medicines.
Let’s consider each of these uses in turn. Perhaps the most familiar use of turmeric is as a colourful, yellow-orange spice, a key ingredient in many curry and Middle-Eastern dishes as well as many chutneys and pickles. The main coloured component is called curcumin, which is also used to colour many other dishes, such as desserts and fish fingers. It has been approved as a food additive and gets listed as E100 on European food labels. Apart from adding colour, the curcumin molecule has also been implicated in preventing food from turning rancid, which it might achieve by reacting more readily than other ingredients (Food Chem. 2006, 98, 720-724)
Turmeric has been used for centuries as a cheap alternative to saffron for dyeing saris and Buddhist monks’ robes yellow (more about this, and other uses, from Kew gardens). It’s colour fades with time, even if mordants are used, but it can also be combined with other colours to dye fabrics in shades of green, brown, scarlet and red (from Wild Colour by Jenny Dean).
Turmeric and its main ingredient, curcumin, have also been investigated for a range of medical uses, often inspired by known applications in traditional medicine. A recent reivew of the scientific literature suggests that while curcumin may have shown some promise in laboratory experiments, it is unlikely to be taken up by the body if taken orally, while some of its chemical properties are likely to give misleading results in trials (J. Med. Chem. 2017, 60, 1620-1637). You can see a range of reports for yourself via these links: Wikipedia, Compound Interest, Nature, Forbes).
2. Curcumin, which also goes by the names diferuloylmethane and (1E,6E)-1,7-Bis(4-hydroxy-3-methoxyphenyl)hepta-1,6-diene-3,5-dione, makes up ~4 % of turmeric powder and is its main active ingredient.

Scheme 1: The two forms of curcumin. Yellow (left) below pH 7.5, shown as the enol form, red (right) at a pH above 7.5.
Curcumin can be used as an indicator of the acid/base character of solutions, as it undergoes a colour change from yellow to red when a basic solution is added (a base has a pH above 7, and often feels soapy – bicarbonate of soda is a relatively weak base, while many kitchen cleaners are stronger). The observed colour change is a result of basic molecules removing a proton (a positively charged hydrogen) from each curcumin molecule (see Scheme 1). This chemical change causes the types of light absorbed by curcurmin to change, thus changing its observed colour to red. If an acid (with a pH below 7, for example vinegar or lemon juice) is then added to the turmeric-containing solution, the colour change can be reversed. While the change is reversible, the curcumin molecules degrade quite easily, deactivating this indicator. This reaction has been covered in one of our Friday Chemistry Fixes.
The removal of a proton from curcumin also affects its ability to dissolve in water (the yellow form is only sparingly soluble), so if you find yourself with a yellow curry stain on a kitchen surface, it is worth spraying it with kitchen cleaner – you may well observe a slight colour change to red and if so, it is likely to wipe away more easily (see this link for technical details).
3. Testing of compounds for medicinal uses relies on either extracting the active compounds in plant-derived mixtures such as turmeric powder (for more about extractions, have a look at our blog post on roses), or in the ability of chemists to prepare each compound in the laboratory. In addition, if a chemical structure is likely to have interesting properties, structurally-related compounds are often also tested; as highlighted in our blog post on chillis, small structural changes can even take the sting out of capsaicin.
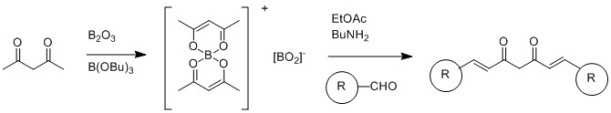
Scheme 2: Pabon synthesis of Curcumin (adapted from Quim. Nova, 2015, 38, 538-552)
The interest in curcumin and related molecules (the family of related structures is called curcuminoids) has led to a range of synthetic strategies being proposed. The most commonly route appears to be based on a methodology proposed by Pabon in 1964 (Pabon, H. J. J., A synthesis of curcumin and related compounds. Recl. Trav. Chim. Pays-Bas, 1964, 83: 379–386.), which relies on the protection of the central acetylacetone as the enol/boron complex, followed by condensation with aldehydes. This approach (Scheme 2, adapted from Quim. Nova, 2015, 38, 538-552) was used by Khan and co-workers to generate a range of compounds for testing as anti-inflammatories (Bioorganic Chemistry, 2012, 40, 30–38). When the aldehyde used is vanillin, this route produces curcumin.
Even though turmeric may not contain a wonder-drug (at least not one which will become bioavailable after ingestion), we hope you have enjoyed this foray into its uses in the kitchen and for dyeing fibres, as well as a reminder of its use as a natural indicator for bases.
Contributors: Jon Shearer, Mike Connolly, Will Glass, Darren Valentine (shared research, writing, images etc.), Natalie Fey (editing, additional content)



