
Tonic water fluoresces under UV light due to the presence of quinine.
1. Quinine is the chemical compound responsible for the ever–dividing bitter taste of tonic water. Tonic water, comprised of carbonated water and quinine, is crucial in a gin and tonic (G&T), but the special chemical properties of quinine mean that it can do much more than act as a mixer on a warm summer afternoon. When exposed to a lamp which emits UV light, by using a violet filter that blocks visible light, the liquid will glow with a brilliant blue colour (above). UV light is also used to visualise the results of thin-layer chromatography and here we used one of these lights on tonic water. The blue is caused by light that is released from the quinine molecules via a phenomenon known as luminescence. Luminescence has practical applications in scientific research and because the amount of light emitted by quinine is precisely known, quinine can be used as a yardstick for other luminescent compounds.
In addition to its scientific usage, quinine has a wide variety of medicinal applications. First used by the Quechua people of Peru, quinine was extracted from the bark of Cinchona (ubescens) trees and used as a muscle relaxant. In the early 17th century the antimalarial properties of quinine were discovered. The first reported treatment of malaria with ground bark of the Cinchona tree, containing quinine, has been attributed to Agostino Salumbrino (see: Caroline Rance – The History of Medicine in 100 Facts, Amberley Publishing, ISBN10: 1445650037, 2015). He was a catholic missionary who came across the medicinal properties while in Peru and then sent a small sample back to the malaria-ravaged Rome. The bark was worth its weight in gold, with the use of quinine spreading throughout Europe in the 17th Century; notably curing King Charles II in the 1670s (Bruce-Chwatt, L J., British Medical Journal (Clinical research ed.), 1988, 296.6635, 1486–1487).
Later, in the 19th century, quinine was purified into a powder and mixed with water and sugar to mask the bitter taste. Only then did British soldiers in India add gin to the concoction, in doing so invent the popular cocktail, although today’s G&T contains much less quinine than the original medicinal recipe!
Quinine continued to be used as an antimalarial until the 1940s, after which alternative treatments for malaria with fewer side effects and better effectiveness were introduced.
2. The brilliant blue colour of a quinine solution under UV light (which is next to visible light in the EM spectrum and possesses a shorter wavelength of 10-400 nm) is due to fluorescence, one type of luminescence. Promotion of the molecule into a higher energy, vibrationally excited state by a photon (a tiny packet of electromagnetic radiation) of UV light is followed by emission of a photon of slightly lower energy. While the molecule is excited, it collides with other molecules and loses energy in these collisions, dropping into lower vibrational energy levels. Eventually, a photon is re-emitted as the molecule drops back into its original state, but, because of the energy loss from collisions, its energy is different and so is the wavelength of the photon, producing a different colour emission to the light which was absorbed. In the case of quinine, this emitted light is the vibrant blue seen above.

Figure 1: The sample holder inside a fluorometer.
Using lamps and light detectors, we can accurately measure the amount of light that is absorbed (“light in”) by a molecule and the amount of light that is re-emitted as fluorescence (“light out”). We do this using a fluorometer (also called fluorimeter), like the one shown above (Fig. 1). By dividing the amount of “light out” by the “light in”, we obtain a value known as the quantum yield. This is a useful figure as it allows us to determine the efficiency of the fluorescence process in a molecule. Quinine is often used a standard molecule to test against, as its quantum yield of 0.55 is well known and so we can calibrate our light detector, as well as our results, against the values we obtain for quinine.
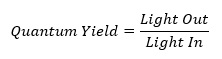 3. Quinine’s antimalarial action is proposed to be like that of another quinolone-based drug, chloroquine. When dissolved in the blood, chloroquine accumulates within the food vacuoles of the malaria parasites in their merozoite form as they infiltrate red blood cells to harvest haemoglobin for essential amino acids (Parasitology Research, 2006, 100(4), 671-676). The breakdown of haemoglobin produces free haem (an iron ion bound to a tetradentate ligand like that shown below, alternative spelling: heme), which is highly toxic and damaging due to its ability to catalyze cross-linking and oxidation in proteins, as well as the formation of lipid peroxides which interrupt cell membranes (Toxicology Letters, 2005, 157(3), 175-188, 2005). Under normal conditions, malarial parasites detoxify the free haem via biocrystallization to form haemozoin, which is not soluble in cell media. However, biocrystallization inhibitors such as chloroquine bind directly to free haem, forming haem-chloroquine complexes that retain toxicity while not being susceptible to biocrystallization. These complexes are also catalytically active, and the catalytic breakdown of the phospholipid bilayer membranes by the formation of lipid peroxides continues, which eventually leads to a rupturing of the cell membrane and cell death.
3. Quinine’s antimalarial action is proposed to be like that of another quinolone-based drug, chloroquine. When dissolved in the blood, chloroquine accumulates within the food vacuoles of the malaria parasites in their merozoite form as they infiltrate red blood cells to harvest haemoglobin for essential amino acids (Parasitology Research, 2006, 100(4), 671-676). The breakdown of haemoglobin produces free haem (an iron ion bound to a tetradentate ligand like that shown below, alternative spelling: heme), which is highly toxic and damaging due to its ability to catalyze cross-linking and oxidation in proteins, as well as the formation of lipid peroxides which interrupt cell membranes (Toxicology Letters, 2005, 157(3), 175-188, 2005). Under normal conditions, malarial parasites detoxify the free haem via biocrystallization to form haemozoin, which is not soluble in cell media. However, biocrystallization inhibitors such as chloroquine bind directly to free haem, forming haem-chloroquine complexes that retain toxicity while not being susceptible to biocrystallization. These complexes are also catalytically active, and the catalytic breakdown of the phospholipid bilayer membranes by the formation of lipid peroxides continues, which eventually leads to a rupturing of the cell membrane and cell death.

Currently, the only economically viable source of quinine is simple extraction from cinchona trees. There are, however, several published syntheses, the first of which was reported by Woodward and Doering (J. Am. Chem. Soc., 1944, 66(5), 849). Woodward, whose work includes the synthesis of many natural products, including cholesterol and chlorophyll, was awarded the Nobel Prize in Chemistry in 1965.
The group of Professor Varinder Aggarwal, based in Bristol, have published a particularly interesting synthetic approach to quinine (J. Am. Chem. Soc. 2010, 132(6), 1828-1830) which uses sulfur ylides (specifically isothiocineole) to shorten a retro-synthetic pathway and allow one-step access to an epoxide pre-cursor of both quinine and quinidine, dependent on the ylide used. Thus, they have created a method to afford asymmetric epoxidation.

While the use of quinine as an anti-malarial is now restricted to cases in which more effective treatments such as ACT (Artemisinin-based combination treatment) is not successful, many strains of malarial parasite have become resistant to quinine.
Economically, the use of cinchona tree bark is unlikely to be usurped by a specialized synthetic route any time soon, particularly as no one wants to be paying sky high prices for a G&T!*
*San Pellegrino also contains quinine for those of you who, like me, despise the taste of tonic water.
Contributors: Huw Powers (initial research, writing, photographs), Tom Young (editing), Derek Durand (editing, structures, images).
An early version of this post was published on Science for All.

