
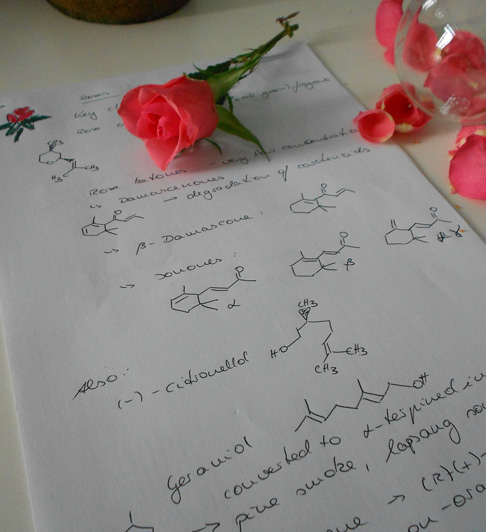
Rose petals and glassware. The molecules shown are rose oxide in the round-bottomed flask and D-limonene in the beaker.
We all recognise the sweet, delicate scent of the rose; that eternal symbol of love, passion and romance. So much so, in fact, that cosmetics companies around the world recreate this exquisite fragrance in their perfumes, lotions and bubble baths. But how can we capture something as intangible and subjective as a scent? And can something created in the lab ever smell as sweet as the real thing?
1. The main image shows rose petals in and around some common laboratory glassware, a round-bottomed flask on the left and a beaker on the right. In the background you can see additional laboratory equipment and a fumehood where a lot of chemical reactions are performed under an extractor fan to prevent the accumulation of fumes and smells in the laboratory. While the roses used for this picture were bought in the supermarket, the Damask Rose (Rosa damascena) is the most common source of rose oil. The molecules shown in the picture are three-dimensional structures of two of the compounds found in the oil extracted from rose petals, rose oxide and limonene.
Many perfumes and other household products incorporate the characteristic scent of roses. This scent can be captured by extracting the aromatic oil from rose petals, but it can also be recreated from some of its key chemical ingredients. Natural rose oil contains a large number of different compounds, many of which have related molecular structures, and here we focus on two interesting examples: limonene (in the beaker) and and rose oxide (in the round-bottomed flask).
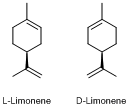
Let’s start with limonene. There are two different ways in which the atoms of limonene can be arranged in space (more about this in section 2), and the two forms have different scents: D-Limonene has a citrus smell, while L-Limonene smells of turpentine. At the molecular level our detection of scent happens because molecules interact with olfactory receptors. These receptors have different shapes, like door locks or the docking stations for different electronic gadgets, and this allows us to identify and distinguish different smells. If you compare the two different forms, they are really just mirror images of each other, so it is rather impressive that we can detect this as very different scents.
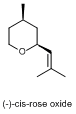
For rose oxide, there are actually four different ways of arranging the atoms in space without changing the order and types of chemical bonds made, and only one of these smells of roses. The other forms have been described as more herbal scents, but are also present in the oil extracted from rose petals. Again, the structural differences are very small, showing just how good we are at distinguishing these molecules.
2. Looking at the structure of limonene, or 1-methyl-4-(1-methylethenyl)-cyclohexene to give its formal name, the two different forms are non-superimposable mirror images of each other and illustrate a phenomenon called chirality. Molecules which are mirror images in three-dimensions might not seem to be very different, but the receptors in our bodies are also chiral, as are many of the other important molecules, and such chiral sites can distinguish between the two forms. In fact, the chirality of the human body is easily visible – just consider your hands or feet as an illustration. Chirality is a big challenge from the point of view of chemical synthesis, because it requires us to control where exactly a reaction takes place on a molecule. Nature achieves this rather easily by providing a reaction environment which is also chiral at the molecular level, and can therefore distinguish between the possible sites for reaction, but it is much harder to achieve in a chemical laboratory.
Rose oxide, tetrahydro-4-methyl-2-(2-methylpropenyl)-2H-pyran, has two chiral centres. In addition, structural changes in this molecule are difficult because of the ring system, and this gives rise to a second difference in three-dimensional arrangement. (We call this stereoisomerism – molecules have the same molecular formula and connections between atoms, but differ in the spatial arrangement of groups.) If you look at the ring structure from the side, the large groups attached to the ring are either on the same side (called the cis form), or on opposite sides (trans). For rose oxide, it is only the (-)-cis form which has a clear rose scent and can be detected at very low concentrations.
3. The extraction of rose oil from petals can use three different approaches: distillation, solvent extraction or extraction with supercritical carbon dioxide. The composition of the essential oil is affected by the temperatures and pressures used during the process and some of the components decompose at higher temperatures (Flavour Fragr. J. 2002, 17, 136-140). While solvent extraction with hexane also extracts waxes and pigments, further purification of the aromatic constituents is considered to produce a superior extract (rose absolute), likely because the lower temperatures prevent decomposition. Extraction with carbon dioxide is possible, because it is reasonably easy to reach the critical point for carbon dioxide at which a supercritical fluid, which behaves like an aromatic solvent, is formed. By returning the extract back to atmospheric pressure, the carbon dioxide disappears, leaving the essential oil (rose absolute or CO2 extract). The process is expensive, but decomposition products can again be avoided.
Rose oil contains a large number of compounds and many of these are closely related structurally. They include rose oxide, the family of rose ketones (damascenones and ionones), as well as limonene, geraniol, various pinenes, benzaldehyde and a range of aromatic alcohols. Only some of these have a scent that is described as rose or floral, while other compounds are present in many flowers and fruit, not just in roses.
Limonene can be synthesised as a racemic mixture by a Diels-Alder reaction, either as the dimerisation of isoprenes, or as the reaction of isoprene with methyl vinyl ketone, the latter followed by a Wittig reaction to covert the ketone into an alkene. An interesting route (Org. Process Res. Dev. 2010, 14, 259-262) for the synthesis of rose oxide involves a singlet oxygenation of beta-citronellol to give a hydroperoxide, which can be reduced with sodium thiosulphate to give a mixture of diols. These cyclise in the presence of acid to give a mixture of stereoisomers. Both compounds are most commonly produced and sold as racemic mixtures, but asymmetric synthesis and resolution are also possible.
Contributors: Natalie Fey (images and text), Simon Perks (photography, editing)



July 11, 2013 at 8:23 am
Reblogged this on Fey Group Webpages and commented:
For something a bit different, check out our new blog over on chempics.wordpress.com.
August 8, 2013 at 9:26 pm
Reblogged this on Plants in my Garden and commented:
From one of my other blogs.
Pingback: Wake up and smell the roses | Cool Story Bromine
Pingback: Onion, Garlic and Chives (Allium spp.) | Picture it...
Pingback: Blogroll: Everyday chemistry : The Sceptical Chymist
Pingback: Eucalyptus spp. | Picture it...
October 25, 2013 at 4:47 am
I am no chemist, but am puzzled about a phenomenon my wife and I noticed the other day. We have several different rose varieties in our new home’s garden, and with the southern spring, they are starting to bloom. Some varieties she can smell, but for me, thre is no perfume, merely a “green” smell on a cut pant. She has always had a stronger sense of smell than I, so I thought little of it. But then I smelled another variety, and appreciated its perfume. Sharing it with her, she could’t smell anything. We found that the rose varieties fell into 3 categories – those she could smell and I couldn’t; those I coud smell and she couldn’t, and those we could both smell. So far, we haven’t found any neither could smell. Do different roses have different chemical bases for their scent? It would seem so, and that she and I do not have all the same receptors as each other. I’d be interested in any research that has been done into this.
February 4, 2014 at 8:51 am
Well, this is a long way out of our comfort zone, but it seems a good theory that there is a difference in receptors or signal processing. Will let you know if I ever come across anything to help explain more formally.
Pingback: Euphorbia spp. | Picture it...
Pingback: Common Yew (Taxus baccata) | Picture it...
Pingback: Spearmint (Mentha spicata) | Picture it...
February 2, 2014 at 7:45 am
Last year I turned 60 and my friend organised an amazing dinner party for me with a Rose/Rosemary theme:
Vodka rose punch with rose bud ice cubes
Rosemary & gruyere cheese straws
Cannellini bean & rosemary on bruschetta
—-
Chilled pea and watercress soup with a rose cream
—-
Roquefort parfait with rosemary honey
—-
Rose & Champagne sorbet
—-
Vegetable gateau with rose harissa & halloumi filling
Roasted shallots and beetroot with rosemary
—-
Poached peaches with rose buds and a rosewater parfait
—-
Coffee/teas
Rose Turkish delight
Rosemary shortbread
Chocolate discs with pistachio & crystallised rose petals
Plus lots of pink champagne…
It was a memorable evening!
February 4, 2014 at 8:49 am
Wow! Sounds lovely.
Pingback: Coming Up Smelling of Roses | Picture it...
Pingback: Workshops with the National Federation of Women’s Institutes | Fey Group Webpages
March 31, 2015 at 2:05 pm
Reblogged this on iktchemie and commented:
Perfekt zum Frühling: welche chemischen Verbindungen machen eigentlich den Rosenduft aus? Nebst tollen Photos wird die Chemie dahinter auch für Laien gut verständlich erklärt.
Pingback: Gelatin | Picture it...
Pingback: Rosemary (Rosmarinus officinalis) | Picture it...
Pingback: Turmeric (Curcuma longa) | Picture it...