Conformers are a type of stereoisomer. They are a pair of isomers that differ from one another by rotation of a single bond. There is an energy barrier involved with rotating around a single bond. If the energy barrier is low, then conformers can interconvert easily between one another and there is so-called “free rotation” about the bond. There can be several conformers of one molecule and usually most molecules will adopt the conformer with the lowest energy.
A technique called NMR (Nuclear Magnetic Resonance), the same technique as used in the hospital for MRI scans, allows us to observe the conformational isomerism of a molecule at different temperatures. At low temperatures, the rotation around a single bond slows down and can even (more or less) stop, affecting the spectrum measured. Computational chemistry can also be used to study rotational barriers, and exploring which conformers are low in energy, and so likely to be present in a reaction mixture, is a challenge for computational studies.
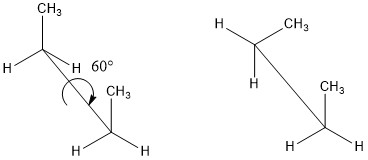
See also glossary entry on chirality for more information on isomerism.
Compiled by Lucy Bird.
