
Oranges spill from a glass beaker; with the molecular structure of D-galacturonic acid, the main constituent of pectin.
In most European countries, the word marmalade refers to all gelled fruit conserves, or what British people might call jam. It’s only in the UK that the name marmalade is reserved for conserves made from citrus fruits. Marmalade features strongly in the Paddington Bear books by Michael Bond which first appeared in 1958 with publication of A Bear Called Paddington. Whilst Orlando the Marmalade Cat appears in 19 children’s books written and illustrated by Kathleen Hale, who died in Bristol in 2000 at 101 years of age. There is something very British about having Seville orange marmalade on your toast for breakfast but how did this culinary tradition evolve and how can chemistry help you create the perfect preserve?
1) The ancient Greeks had recipes for a preserve of quince and honey, which was used as a medicine as well as a sweetmeat. The Greek name mēlomeli passed into Portuguese as marmelo, their word for quince. A conserve of quince and sugar called marmelada was imported from Portugal in the 16th century and considered a luxury food in the UK, eaten as a sweetmeat at the end of meals and believed to be an aphrodisiac. It was very much valued as a medicine, mainly as an aid to digestion. Marmalade “is very good to strengthen the stomach, and to keep the meat therein till it be perfectly digested” [William Langham, The Garden of Health 1579].
There is a story that Mary, Queen of Scots, introduced orange marmalade into Britain. It seems she was given medicinal marmalade either when she suffered from sea sickness on a journey from Calais to Scotland in 1561, or when she was sick at Jedburgh. She responded with “Marmalade pour Marie malade” or possibly “Marmalade pour ma maladie”. But this probably refers to quince marmalade which was more common then for medicinal use.
In the Middle Ages, Arabs had introduced oranges, lemons and sugar into southern Europe. These oranges were bitter and their sharp, sour juice was enjoyed in cooking, while the peel could be conserved by drying or stored in syrup. During the 16th century, the techniques for making marmalade from quinces became better known and were applied to oranges too, and so by the 17th and 18th centuries various recipes for orange marmalade appeared in handwritten and printed cookery books. It still tended to be used at the end of meals and medicinally to “warme the stomack, digest, and breake winde” [Robert Lovell, A Compleat Herball, Oxford, 1665].
The Scottish contribution to the history of marmalade came in the early 18th century when marmalade started to appear on Scottish breakfast tables. Scottish breakfasts became famous and fashionable, so orange marmalade with a softer consistency, which could be spread on toast or oatcakes, became widespread in England too. By the 19th century, rather than a sweetmeat for dessert, it was popular as a breakfast spread.
Scotland can also be credited with the first commercial production of marmalade. James Keiller, a grocer in Dundee, bought a cheap load of Seville bitter oranges from a ship sheltering from a storm in Dundee harbour. His wife Janet turned them into marmalade, which sold so successfully that the company James Keiller & Son was soon established, to manufacture and sell marmalade commercially. With a high sugar content marmalade travels well and withstands extremes of temperature, so these companies ensured it became popular in overseas countries associated with Britain during the 19th century as one of our traditional national foods.
2) It is pectin which is responsible for the firm texture of jellies and jams. Quince belongs to the same family as apples and pears and is very high in pectin. It is not very common now in Britain although it can be grown here. Foodies are rediscovering it and you might be familiar with membrillo, a quince paste popular with cheese in Spain. Seville oranges are also very high in pectin, especially in the peel and pips. You can observe the pectin in Seville orange pips by putting them in water and leaving them in the fridge overnight – the next morning you will have a very viscous jelly; this is a really useful pectin stock for adding to any preserves you might be making.
Pectin is a long chain of molecules joined together. This chain of molecular units is known as a polymer – poly, meaning many. We are surrounded by examples of polymers; man-made, or synthetic, polymers can be found in plastic bags and plastic windows or doors, as well as nylon stockings and shirts. Whilst naturally occurring, or bio-polymers, include silk and DNA, as well as pectin. One of the molecules, which is repeated in the chains of pectin, is D-galacturonic acid. This is a cyclic molecule, which has both carbons and an oxygen in the ring, as well as three attached alcohol groups and a carboxylic acid. D-Galacturonic acid is a type of sugar with a very similar structure to fructose and glucose; these sugar molecules are often referred to as saccharides, and thus pectin is a polysaccharide.
Carbon and oxygen have very different electronegativities. The oxygen atom has slightly greater electron density than carbon; this is also true of the oxygen-hydrogen bonds, where the oxygen is slightly more negative and the hydrogen slightly more positive. This difference in polarity across the carbon-oxygen and oxygen-hydrogen bonds allows pectin to interact favourably with water molecules, which consist of hydrogen-oxygen bonds. Unlike many synthetic polymers, this favourable interaction with water allows pectin to be soluble in water, even though it is a very long molecule; hence we can extract the pectin from fruit pips into water.
3) Although pectin was discovered over 200 years ago, the composition and structure of pectin are still not completely known. Whilst rich in galacturonic acid, this is not the only molecule which makes up pectin and several different polysaccharides have been identified. Homogalacturonans, linear chains of galacturonic acid are known, as well as repeating disaccharide units of galacturonic acid and rhamnose. Many of these polysaccharide chains are substituted with side-chains of neutral sugars, including D-galactose, L-arabinose and D-xylose; the proportions of each varying with the origin of the pectin (D. G. Oakenfull, The Chemistry and Technology of Pectin, 1991, New York Academic Press; see also Plant Cell Walls, University of Georgia, Ccrc.uga.edu, retrieved on 4th February 2013).
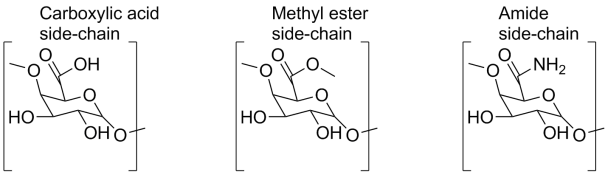
The various side chains resulting from decomposition in storage, commercial isolation or as a result of the different natural sources for pectin.
The structure of pectin is difficult to determine because pectin can change during isolation or whilst being stored (Chemistry of Natural Compounds, 2000, 36, 1 – 10). In nature, around 80 % of the carboxylate groups of galacturonic acid are esterified with methanol. Although, in sugar beet, potatoes and pears, the side chains are more commonly acetylated. Pectins are commercially treated with ammonia, resulting in a carboxamide group. The ability of pectins to form gels, to make your marmalade firm, rather than sloppy, depends on the molecular size of the pectin and on the degree of esterification; thus different pectins are not equally able to form gels.
Importantly, for jam and marmalade makers, is the relative amount of esterification of the pectins present. High ester pectins, containing lots of esterified groups, are soluble only at very acidic pH (typically pH 2.8 – 3.6), whereas low ester pectins, with more carboxylate or amide side chains, are not as sensitive to pH levels. So, it is important to have an acidic, aqueous solution when making marmalade – this shouldn’t be too problematic, as the oranges themselves contain high quantities of citric acid, and lemon juice is often added to jams. However, solubilised pectins are also sensitive to degradation in very acidic solution, especially at elevated temperature. This is because the acidic conditions allow hydrolysis of the glycosidic linkages, resulting in chain cleavage and loss of gel forming properties. So, the marmalade should not be heated for too long.
The final important stage is, of course, the formation of the jelly-like consistency which allows us to spread the marmalade on our toast. The carboxylate groups exist as anions, often with calcium counter ions. These anionic group serve to aid water-solubility, since the polymer chain is highly hydrated, and also keep pectin chains relatively straight, since the charges repel each other (S. Paoletti, Chemistry and function of pectins, 1986, Washington DC, American Chemical Society). When the pH is lowered, the carboxylate groups are protonated and are no longer charged; this allows the pectin chains to aggregate and form a gel. Note that, since desterification is also favourable under low pH conditions, even high ester pectins will become more soluble, and more able to form gels, at higher pH.
While the sales of commercial marmalade seem to be falling, more people want to make their own marmalade. Pectin has been found to be a dietary fibre, binding to cholesterol and slowing glucose absorption. Indeed, consumption of pectin has been shown to reduce blood cholesterol levels (Silpakorn University International Journal, 2003, 3 (1 – 2), 206). So, marmalade is not only delicious but may also have dietary benefits. Seville oranges are available in January/February, so why not have a go?
Contributors: Rose Silvester (research, words, photos), Jenny Slaughter (research, words, photos). Our thanks to Lionel Hart for providing us with numerous oranges.





