The word chirality is derived from the Greek word for hand. Our hands are chiral objects as they are mirror images, but you can’t align them by putting one on top of the other. This explains why left-handed people need different scissors to right-handed people.
Molecules can also be chiral, and a chiral molecule is not symmetrical. It cannot be superimposed on (put on top of) its mirror image. The molecule below (bromochlorofluoromethane) is chiral.
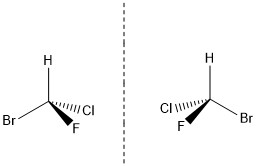
Chiral molecules are an example of stereoisomerism. Stereoisomers are isomers that have the same molecular formula and their atoms are bonded in the same order but the spatial arrangement of their atoms are different. There are two main types of stereoisomer: enantiomers and diastereomers. Enantiomers are also known as optical isomers, they are non-superimposable mirror images (see the molecule above), which have the same physical and chemical properties, unless they encounter other chiral molecules.
Diastereomers have several chiral atoms, but are not mirror images of each other. Diastereomers have different physical and chemical properties, whereas enantiomers only differ by one property: their interaction with plane-polarised light. One enantiomer will rotate plane-polarised light clockwise and the other enantiomer will rotate plane-polarised light anticlockwise by an equal amount to the other enantiomer. If you have a racemic mixture (equal amount of both enantiomer) of two enantiomers there will be no effect on plane-polarised light as the rotations cancel.
Note that this summary has focussed on centrochirality – there are other types of chirality, too.
Compiled by Lucy Bird.
