Aloe is a genus containing over 500 species of flowering succulent plants. These plants have not only been used as aesthetically pleasing house plants but also for their numerous pharmaceutical properties. In this post we will take a look into where colonies of this genus can be found and how these dynamic plants are used in modern day science.
1) The Aloe genus is native to Africa. In particular, colonies can be found in southern Africa, the mountains of tropical Africa and various islands off the coast of Africa (www.theplantlist.org). The genus is placed amongst the family Xanthorrhoeaceae and subfamily Asphodeloideae (www.mobot.org). Of the 500 species making up the genus, the most commonly known is Aloe vera. Aloe vera no longer exists in naturally occurring populations but has been cultivated for its pharmaceutical properties (nccam.nih.gov/health/aloevera).
The picture above shows an Aloe plant from which two leaves have been cut and placed in a petri dish. Petri dishes have many different uses in the chemistry laboratory. They might be used in a traditional way, to grow cell cultures of certain bacteria. Or, they might be used as a container for drying solids, such as crystals, isolated from a process. Behind the Aloe plant is a glass funnel, another frequently used piece of apparatus in laboratory work. In the plant pot is a molecule of aloin; a compound found in aloe which chemists are still struggling to make. Aloin has a flat set of fused rings at the top and a single sugar-type molecule suspended beneath.
Aloe vera can form two substances, gel and latex, which are used for medicinal purposes. Aloe gel is the clear, jelly-like substance found in the inner part of the plant leaf. Aloe latex comes from just under the plant’s skin and is yellow in colour. Both the gels and latexes have been used in herbal medicine for the last 2000 years and now these extracts of Aloe vera are frequently used in cosmetics and health industries, being marketed as having rejuvenating, healing and soothing properties. Aloe possesses cooling properties, similar to menthol, making it a great way to soothe burns.
Tissues are completely destroyed when burnt (www.britannica.com), unlike other wounds. Burns are also accompanied by inflammation and a serious risk of bacterial infection. So what makes Aloe vera so great at healing burns? It has been reported to inhibit the multiplication of bacteria, viruses and fungi, thereby preventing infection. In addition, it is thought to work as an anti-inflammatory agent, to relieve swelling, and relieve pain and itching (timeforaloe.com). However, the positive scientific evidence for the medicinal effectiveness or safety of Aloe vera extracts, for either cosmetic or medicinal purposes, is frequently contradicted.
2) Despite controversy over the effectiveness of aloe extracts, scientists have found 150 beneficial components in Aloe vera. There does not seem to be one magic ingredient in particular; it is more likely that multiple components work in conjunction to create healing and health giving benefits. The ten main chemical constituents of Aloe vera include: amino acids, anthraquinones (see Rhubarb, July 2013), enzymes, minerals, vitamins, lignins (see Miscanthus, July 2013), monosaccharide, polysaccharides, salicylic acid, saponins, and sterols. So, Aloe vera is full of potential useful compounds. How might these ingredients be beneficial for the human body?
In order to fully function, the human body requires 22 amino acids, of which 14 can be made by enzymatic provesses. Ingesting food and drink is the way the body receives the other eight essential amino acids. All of these essential amino acids are available in Aloe vera. The body uses amino acids to build proteins for a variety of jobs, such as for growth and repairing cells (Why do we need amino acids, ChemInfo – Chemistry Blog).
Amino acids are generally classified due to the properties of their side-chains. The side-chain can make an amino acid a weak acid or a weak base. The amino acid will be hydrophilic, “water-loving”, if the side-chain is polar; meaning it will be soluble in water. Or hydrophobic, “water-hating”, and thus less soluble in water, if the side chain is non-polar (Thomas H. Creighton, 1993 “Chapter 1”. Proteins: structures and molecular properties. San Francisco: W. H. Freeman).
Of the standard α-amino acids, all but glycine can exist in either of two enantiomers, called L or D amino acids. Enantiomers are mirror images of each other due to the chiral α-carbon; this is a carbon centre with four different groups around it, which can be arranged in two different ways.

A zwitterionic amino acid; the amine residue has a positive charge and the carboxylate has a negative charge.
The amine and carboxylic acid functional groups found in amino acids allow them to have amphiprotic properties, meaning they can have both acidic and basic properties (Thomas H. Creighton, 1993 “Chapter 1”. Proteins: structures and molecular properties. San Francisco: W. H. Freeman). In basic solutions, COOH, deprotonation occurs and the negative carboxylate ion, COO–, predominates. Whereas, in acidic solutions, protonation occurs and the positively charged ammonium ion, NH3+, predominates. Between these two pH values a structure with net zero charge called a Zwitterion forms (William J. Simmons and Gerhard Meisenberg, 2006, Principles of medical biochemistry. Mosby Elsevier, p. 19); where there is both a negatively charged carboxylate ion, COO–, and a positively charged ammonium ion, NH3+, present.
In Aloe vera there is also an abundance of enzymes (Indian J. Dermatol., 2008, 53 (4), 163 – 166). Enzymes act as biochemical catalysts, to provide fuel for cells in the body. Some of the main enzymes, and their functions, in Aloe vera include:
- Amylase – which breaks down sugars and starches;
- Bradykinase – which stimulates the immune system, and is both analgesic and anti-inflammatory;
- Lipase – which aids in the digestion of fats.
Aloe vera, an anti-oxidant rich plant, contains many vitamins such as A, C, and E plus the minerals, zinc, and selenium (Molecules, 2013, 18 (5), 4943 – 4954). These anti-oxidants can help to boost the immune system and combat free radicals in the body. Whilst it isn’t clear how these compounds would be accessible to the body, if using an aloe hand cream, for example, it is certainly the case that Aloe vera contains many compounds of interest to the chemist.
3) On the inner lining of Aloe plants is a yellow resinous substance known as aloin. Aloin is a bitter, yellow-brown coloured anthraquinone noted in the exudate of at least 68 Aloe species at levels from 0.1 to 6.6% of leaf dry weight. It is anti-bacterial, anti-viral, and analgesic (Dr. Peter Atherton, Aloe Vera Myth or Medicine?, PositiveHealthOnline, May 1997). Aloin’s main use is as a stimulant-laxative, treating constipation by inducing bowel movements.

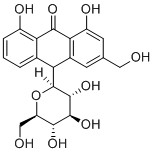
5-hydroxyaloin A; the thiomethyl moiety has proved useful as a handle for synthesis yet an insurmountable challenge to remove.
Aloe-derived 5-hydroxyaloin A, has not yet been achieved by total synthesis and it is instead extracted directly from the natural source (Donald G. Barceloux, Medical Toxicology of Natural Substances: Foods, Fungi, Medicinal Herbs, Plants, and Venomous Animals). Synthesis of aloin derivatives could potentially uncover some interesting uses for these compounds in the pharmaceutical industry. A thiomethyl 5-hydroxyaloin A has been synthesised to provide a new route to forming, the often challenging, C-glycosidic linkage. (Phytochemistry, 2000, 55, 949–952). This is of interest because the C-aryl glycoside class of natural products have significant anti-cancer properties. (Pure App. Chem., 1994, 66 (10/11), 2135-8). The synthesis of thiomethyl 5-hydroxyaloin A was achieved using benzyne and naphthyne [4 + 2] cycloadditions with substituted furans.
The aim of the experiment was to synthesise 5-hydroxyaloin A but this failed as attempts to desulfurize the thiomethyl group using a variety of reagents, including deactivated Raney-Ni, nickel boride, and other nickel-containing complex reducing agents, such as NiCRA (J. Org. Chem., 1989, 54 (20), 4848 – 4853), were uniformly unsuccessful, giving either no reaction or intractable mixtures (Org. Lett., 2010, 12 (24), 5632–5635). The thiomethyl group did however direct the regiochemistry of the ring opening of the cycloadduct to the desired C-aryl glycoside.
Further research into some of the components and derivatives of the aloin components of aloe may open new doors into other pharmaceutical properties of this plant.Contributors – Sam Montgomery (research, ideas and words); Isabel Perez-Powell (ideas and research); Jenny Slaughter (photo, computational structures and editing).





![The [4+2] cycloaddition, which was key to the synthesis of 5-hydroxyaloin A.](https://chempics.files.wordpress.com/2014/02/4plus2cycloadditionscheme.jpg?w=611)