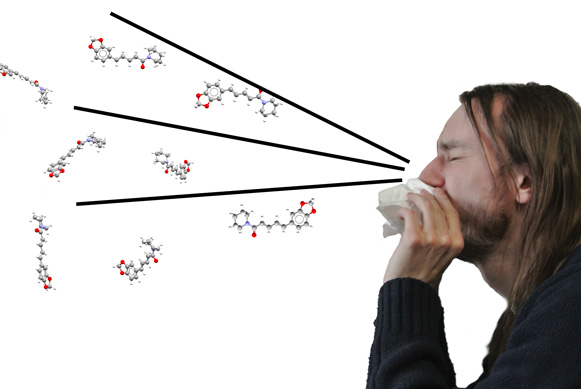
Simulating not just piperine molecules, but a sneeze…
Black pepper is a staple spice in our diet, found on dining tables across the country. But what makes this spice so diverse? Why does it make us sneeze? And what are its effects on digestion? We delve deep down to the molecular level and report our findings.
(This post has been prepared by students from the Theory and Modelling in the Chemical Sciences Centre for Doctoral Training, who are currently doing their PhD research in Oxford, Southampton and Bristol.)
1. Grown mostly in tropical regions like Southern India and Vietnam, dried black pepper (Piper nigrum) has been used as a spice all over the world for millennia. As well as seasoning food, pepper has a rich history as a medicinal remedy, applied to everything from eye infections to sunburn. But what do we know about it today?
The peppercorns we grind up and put in food are made up of a variety of components, such as fiber, essential oils, and minerals. But the source of many of its properties is the molecule piperine, shown in the main image, which belongs to a class of compounds known as alkaloids – more about these compounds below. These molecules are usually extracted from plants, and are known to cause significant reactions in the body. Piperine is what gives pepper its bitter taste and it’s also responsible for making the spice an irritant – this is what makes us sneeze!
An irritant is a chemical that is not corrosive but produces inflammation in living tissue at the site of contact. When breathed in, piperine appears to irritate the membrane that produces mucous inside the nose. As well as the chemical side of things, grinding peppercorns to a fine powder makes it easy for particles to land inside the nose and trigger nerve cells. The contact with these nerve endings seems to make us sneeze. However, the exact mechanism by which piperine causes irritation of mucous membranes is still not understood.

Cracked pepper corns spilling out of a plastic test tube.
Aside from sneezing, there have been numerous studies of piperine’s physiological effects on the digestive system. Just as with mucous membranes in the nose, pepper can cause irritation in the stomach. It has also been found to increase, for example, the effect of digestive enzymes such as amylase (which digests carbohydrates) and lipase (which digests fats). On the other hand, the molecule has been found to inhibit drug-metabolising enzymes. This slows the breakdown of active compounds in drugs, increasing their availability to the body. The mechanism of this increase is, as of yet, poorly understood.
2. Alkaloids are found to occur naturally and usually contain a nitrogen atom in a ring which is able to accept protons, i.e. it acts as a base. Many different molecules are classed as alkaloids, which is an old-fashioned way of naming them (this wikipedia entry explains this further), and more recent attempts at grouping these molecules rely on the structural framework and functional groups present. An overview of naturally occuring compounds containing nitrogen is given here, and the naming of heterocycles, i.e. structures containing non-C atoms in rings, is outlined here. As you can see, other N-containing heterocyclic structures first described as alkaloids include morphine, caffeine and nicotine, all covered in earlier posts and shown in Figure 1, although they are not very closely related to the structure of piperine. Structural similarity can lead to similar interactions in the body, something we explored in our post on chillis.
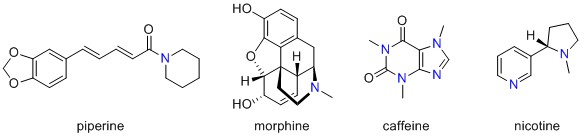
Molecules described as alkaloids
Despite pepper being part to our diet for many centuries, this common spice still retains its mystery. And although the folklore of cures for all kinds of ailments (see this page from the Royal Botanic Garden at Kew for an overview) have not all stood up to modern science, the mechanisms for how we react to it remain elusive. But by isolating the active ingredient of many of its properties, chemistry is now beginning to shed light on how biological systems respond to piperine.
Peppercorns contain a mixture of compounds and to study piperine, chemists need to isolate the molecule, which can be done either by extracting it from black pepper or by chemical synthesis. A number of different approaches to extraction have been described (J. Chem. Educ., 1993, 70 (7), 598-599), which all rely on differences in solubility and volatility of the different compounds present. The most commonly described extraction process (referenced to Ikan R (1991). Natural Products: A Laboratory Guide 2nd Ed. San Diego: Academic Press, Inc. pp. 223–224. ISBN 0123705517) begins by mixing ground black pepper with ethanol. The resulting mixture is then filtered, and a little potassium hydroxide in isopropyl alcohol is added to the remaining residue to remove any resin. Once a fluid separates along the top of this mixture, this is decanted and left overnight so that piperine crystals can form.
3. The piperine content of black pepper is between 6-9% but often only 2-4% can be extracted, so chemical synthesis is preferred. One such synthesis, described in detail here and more formally in this research paper (J. Agric. Food Chem., 1981, 29 (5), 942–944), uses a Wittig type reaction to form the conjugated trans-alkene.
A possible reaction pathway is shown in Scheme 1 below: A phosphonate ester is prepared by the reaction of triethylphosphite with a brominated ester. The phosphonate ester reacts under basic conditions as a phosphonium ylide with an aldehyde to form a conjugated trans ester with the desired stereochemistry. The methoxy moiety of the ester group is then substituted with piperidine to form the special molecule that gives pepper its bite.
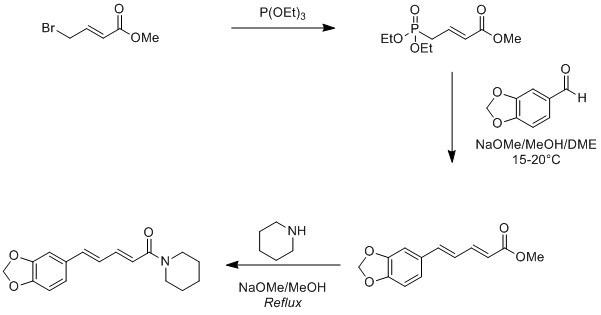
Scheme 1
As you can see, regardless of whether you extract piperine from peppercorns or make it in the laboratory, we can use it in isolation to better understand its structure, properties and uses, as well as determining how biological systems respond to piperine. And as we improve our understanding of these interactions, we might yet uncover the secrets behind this king of spices.
Contributors: Silvia Amabilino, David McDonagh, Staszek Welsh, Ian Shepherd (shared research, writing, images etc.), Natalie Fey (editing).

