 Week 8 of the Bakeoff was the historically themed Tudor week, in which the bakers were required to create sweet and savoury foods that might have graced the table at a banquet five hundred years ago. The signature challenge was to make shaped pies; some bakers opted for traditional fillings such as game, while others took a more modern approach to their flavour combinations. We saw in some detail the processes being used to make the pie crusts, which had to contain the fillings, hold their own shape and of course taste good themselves. A particularly striking moment was a discussion of the amount of lard used to make “hot water crust pastry”, so let us have a more detailed and scientific look at the way in which fat alters the production of gluten, from flour and water, to give pastry its texture and properties.
Week 8 of the Bakeoff was the historically themed Tudor week, in which the bakers were required to create sweet and savoury foods that might have graced the table at a banquet five hundred years ago. The signature challenge was to make shaped pies; some bakers opted for traditional fillings such as game, while others took a more modern approach to their flavour combinations. We saw in some detail the processes being used to make the pie crusts, which had to contain the fillings, hold their own shape and of course taste good themselves. A particularly striking moment was a discussion of the amount of lard used to make “hot water crust pastry”, so let us have a more detailed and scientific look at the way in which fat alters the production of gluten, from flour and water, to give pastry its texture and properties.
Pastry is an ancient tradition of cookery all over the world. The ancient Egyptians, Greeks and Romans are all thought to have eaten small pastry delicacies, and well-known East Asian pastry goods include filled steam buns and festival foods like “mooncakes”. European pastry cooking as we know it developed during the mediaeval period and perhaps came to the fore with the success of the first ever celebrity chef – Marie-Antoine Carême (1784-1883).

Flour on a weighing scale
While his delicate creations were eaten at the tables of European high society, common people had their own ways of putting pastry to use. One of the most famous examples in Britain is the Cornish pasty – this miners’ food made use of pastry to transport cooked meat and vegetables, and it could be eaten while holding the crust to prevent the food from being spoiled by dirt.
Nowadays a vast range of pastry goods can be bought or baked at home, made with many different sorts of pastry. Quiches and tarts are made with shortcrust pastry, while many ornate patisserie sweets can be made with lighter pastries such as puff or choux. A considerable range of textures can be achieved with different kinds of pastry, but the key ingredients are the same.

Butter and lard – two fats used in pastry making
In the first step of making pastry the fat, or “shortening”, is combined with the flour before water is added; the fat may be butter, vegetable shortening (made from hydrogenated vegetable oils) or, in the case of hot water crust, lard (pig fat) melted in hot water. This gives pastry its firm, crumbly texture which contrasts with sticky, stretchy dough made by mixing flour and water. It also helps to prevent the filling from leaking out through the pastry during cooking as water and fats do not mix well.
The microscopic origin of the very different textures of dough and pastry lies in the formation of gluten. Gluten is a protein-based substance, made when the proteins in flour mix with water, which gives bread its elasticity.
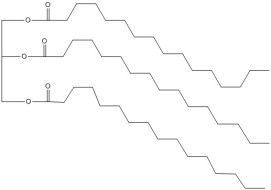
Palmitin – an example of a triglyceride
Fats, like butter and lard, are hydrophobic, since they are made from molecules called “triglycerides” which contain long hydrocarbon chains, so by rubbing flour and butter together the particles of flour are coated with a water-repellent layer. This significantly reduces the extent to which gluten is formed and leaves the pastry firm rather than stretchy. If pastry is overworked then longer strands of gluten are produced and it becomes tough; when making bread, on the other hand, the aim of kneading is to make these long strands of gluten to strengthen the dough.
Gluten is a network of hundreds of protein components of various sizes and chemical characters – this lack of definition makes it hard to describe exactly what gluten is. The components are traditionally separated into “glutenins”, which give dough its strength and elasticity, and “gliadins”, which impart viscosity and extensibility. A much more thorough description is given in this paper. Perhaps the most important distinction between the two is size – gliadins are monomeric (single, if very large, molecules) whereas glutenins are vast aggregates of many proteins connected by crosslinking bonds. An important type of crosslinking is that done by disulphide bonds made by oxidation of –SH groups in the protein; these are the same bonds which give hair and vulcanized rubber their physical strength.
The glutenins are also held together by other sorts of covalent crosslinks, such as those between tyrosine residues, and by intermolecular forces like the attraction between oppositely charged parts of different molecules.
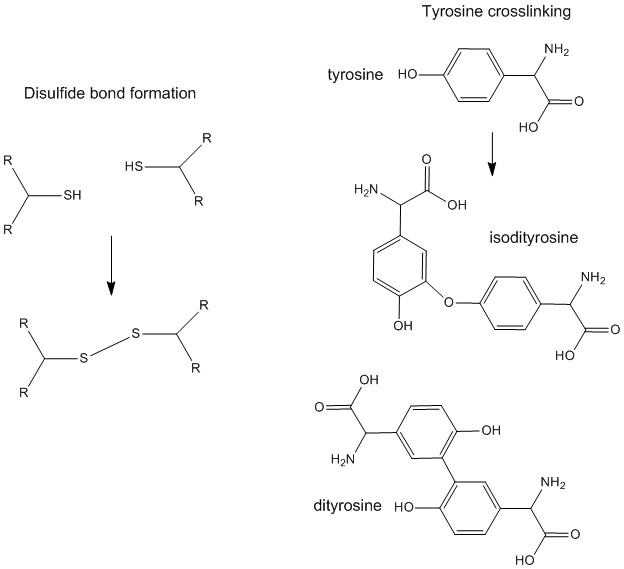
Two types of crosslinking between proteins in gluten
The chemistry of pastry is still not understood in detail, yet it has been expertly exploited by bakers for millennia. With patisserie week fast approaching in the Bakeoff tent we will be sure to see more pastry-making techniques on display soon.
