Raspberries and a molecule of raspberry ketone (4-(4-Hydroxyphenyl)butan-2-one) in a mortar, with the pestle lying next to it.
In the UK and most of Western Europe, our traditional autumn recipes are dominated by apples, blackberries and later pears, but the autumn-fruiting cultivars of raspberries, such as Rubus idaeus ‘Autumn Bliss’ have also withstood all that your average British summer could throw at them and appear bright red, like little burlesque dancers, in the fading colours of the productive garden. So “blow a raspberry” to all the more exotic fruit marketed (often misleadingly) as “Superfood” and pay homage to this potent little package of vitamins, minerals, antioxidants and fibre.
1. The main picture shows a few raspberries in a mortar, together with the pestle lying next to it, as well as a molecule of raspberry ketone, the compound giving rise to the characteristic aroma of raspberries and other berries. Mortar and pestle are often used in the laboratory to crush crystalline solids into smaller particles; you can even carry out chemical reactions by grinding. They are also useful in the kitchen for crushing herbs and spices, making pastes by combining these with a few drops of oil, or even for preparing dips and sauces, such as pesto and guacamole. And if you want your raspberry jam to be seedless, you might crush and then sieve your berries to extract the juice and pulp, although hopefully you would make more jam than might fit into a small mortar. Since you need a lot of raspberries to extract useful amounts of raspberry ketone (Wikipedia quotes 1-4 mg per kg of raspberries), the natural flavour is very expensive and it is cheaper to prepare it synthetically – more about this below.
Raspberries belong to the rose family, Rosaceae, and they are perennials that grow on woody stems. The main cultivars used in Europe fall into two groups: summer-fruiting, which bear fruit on growth produced in the previous season, and autumn-fruiting, which produce berries on the same season’s growth. (For more horticultural information, have a look at this RHS page.) They like quite wet summers, and indeed many of the fruit sold in UK supermarkets are grown in Scotland (see the Berry Scotland Programme for some more background). You might not realise it, but what we tend to think of as a single raspberry fruit is actually a cluster of so-called drupelets (a drupe is a fruit where the flesh surrounds a shell which contains a seed), making it an aggregate fruit. Since each drupelet contains a seed, this contributes to the high fibre content of raspberries; this varies, but can be as high as 6% (see Nutrition Facts). Apart from the fibre, raspberries are also quite high in Vitamin C and contain a number of polyphenol antioxidants (see for example Food Chemistry, 2014, 156, 362–368 and Food Chemistry, 2014, 160, 233–240), i.e. compounds thought to remove free radicals and so prevent their reaction in cells, which may cause damage. Note that it is not always clear how much of the antioxidant properties survive digestion, though (for a brief summary, see the entry on Wikipedia, for a bit more, try this link). Similarly, the slimming properties of raspberry ketone have not exactly stood up to scrutiny. Still, raspberries will grow in the wettest of summers, provide you with vitamins and fibre, as well as tasting great both fresh and cold, so they get my vote any day.
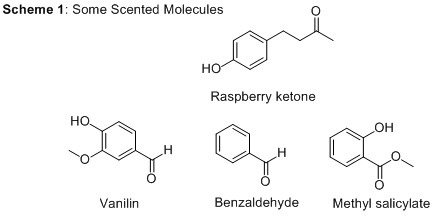 2. Raspberry ketone,* or 4-(4-Hydroxyphenyl)butan-2-one, (shown both in the main image and in Scheme 1) is a key component of these powerful little (aggregate) fruit and has many uses in the cosmetic and food industry. Ketones have a carbonyl group (C=O) with carbon-containing groups on either side; in the case of raspberry ketone, one of these groups also contains a phenol group, consisting of a benzene ring and an OH group. Many scented molecules contain both aromatic and carbonyl groups, and Scheme 1 also shows the structures of vanillin (see our post on Vanilla), benzaldehyde (which smells like almonds) and methyl salicylate (smelling of mouthwash and related to acetyl salicylic acid, see our post on Aspirin), highlighting how quite small structural changes can be detected by our noses as very different scents. While the carbonyl group is present in all of these molecules, its neighbours change and need to be taken into account when naming the functional groups present. For vanillin and benzaldehyde, the carbonyl groups thus form part of an aldehyde, whereas for methyl salicylate, it forms part of an ester.
2. Raspberry ketone,* or 4-(4-Hydroxyphenyl)butan-2-one, (shown both in the main image and in Scheme 1) is a key component of these powerful little (aggregate) fruit and has many uses in the cosmetic and food industry. Ketones have a carbonyl group (C=O) with carbon-containing groups on either side; in the case of raspberry ketone, one of these groups also contains a phenol group, consisting of a benzene ring and an OH group. Many scented molecules contain both aromatic and carbonyl groups, and Scheme 1 also shows the structures of vanillin (see our post on Vanilla), benzaldehyde (which smells like almonds) and methyl salicylate (smelling of mouthwash and related to acetyl salicylic acid, see our post on Aspirin), highlighting how quite small structural changes can be detected by our noses as very different scents. While the carbonyl group is present in all of these molecules, its neighbours change and need to be taken into account when naming the functional groups present. For vanillin and benzaldehyde, the carbonyl groups thus form part of an aldehyde, whereas for methyl salicylate, it forms part of an ester.
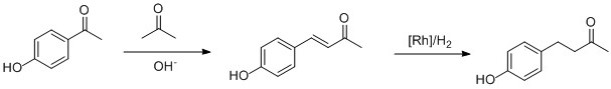
3. Since extraction of raspberry ketone from raspberries is costly (just think of how many raspberries need to be picked, see above), many synthetic routes for its production have been developed, with some recent routes involving heterogeneous palladium catalysis (Adv. Synth. Catal. 2007, 349, 1949 – 1954) and continuous flow reactors (Org. Process Res. Dev. 2011, 15, 858–870). Another route, described as “suitable for the introductory chemistry laboratory”, has also been proposed (The Chemical Educator, 1996, 1, 1-18, see also this link) and is shown in Scheme 2.
This reaction involves a crossed aldol reaction between 4-hydroxy benzaldehyde and acetone, which is followed by the rhodium-catalysed hydrogenation to give the desired product in high yield and with good purity. With such synthetic routes in hand, we can safely stick to eating the glorious fresh fruit or using it in jam, while leaving the production of raspberry ketones for flavours and scents to industrial chemists.
Contributors: Natalie Fey (research, words, photos and images), Jenny Slaughter (editing).
*See also a post by our Bristol colleagues over at Molecule of the Month.